Ammonia surrogates in the synthesis of primary amines - Organic & Biomolecular Chemistry (RSC Publishing)
4.6 (783) · $ 17.50 · In stock
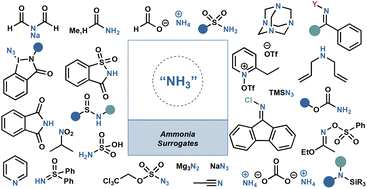
Primary amines are derivatives of ammonia in which one hydrogen atom is replaced by an alkyl or aryl group. Ammonia serves as the primary nitrogen source in amination reactions, and its utilization in solution or as a pure gas has witnessed notable advancements. However, the use of gaseous ammonia remains pr

Coupling of Methyl Ketones and Primary or Secondary Amines Leading to α-Ketoamides
Pharmaceuticals, Free Full-Text

N,N-Dialkyl Amides as Versatile Synthons for Synthesis of Heterocycles and Acyclic Systems
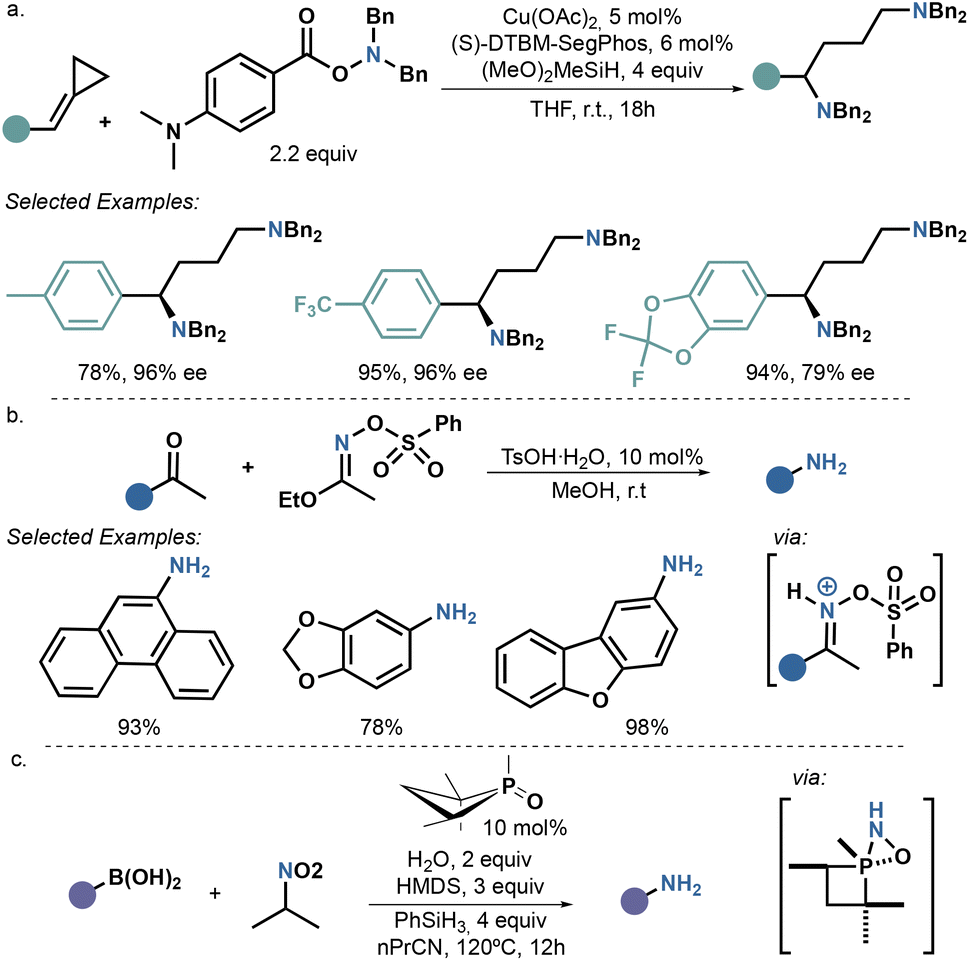
Ammonia surrogates in the synthesis of primary amines - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D3OB01202F
Ammonia surrogates in the synthesis of primary amines - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D3OB01202F

Ammonia surrogates in the synthesis of primary amines - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D3OB01202F

Tritylamine as an Ammonia Surrogate in the Ugi Reaction Provides Access to Unprecedented 5-Sulfamido Oxazoles Using Burgess-type Reagents
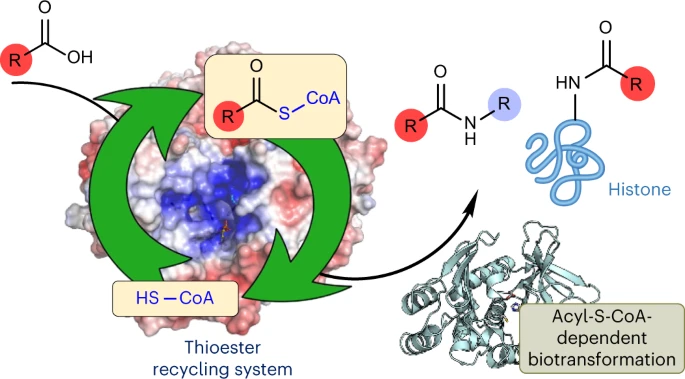
Publications – Turner Biocatalysis
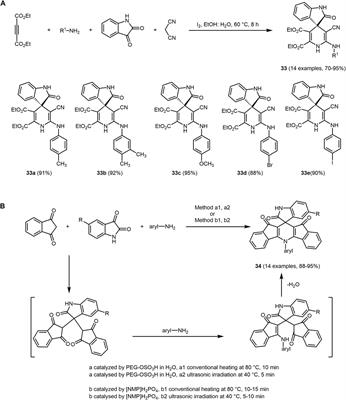
Frontiers Multicomponent synthesis of chromophores – The one-pot approach to functional π-systems

Carboxylic Acid Bioisosteres in Medicinal Chemistry: Synthesis and Properties

N,N-Dialkyl Amides as Versatile Synthons for Synthesis of Heterocycles and Acyclic Systems

N,N-Dialkyl Amides as Versatile Synthons for Synthesis of Heterocycles and Acyclic Systems
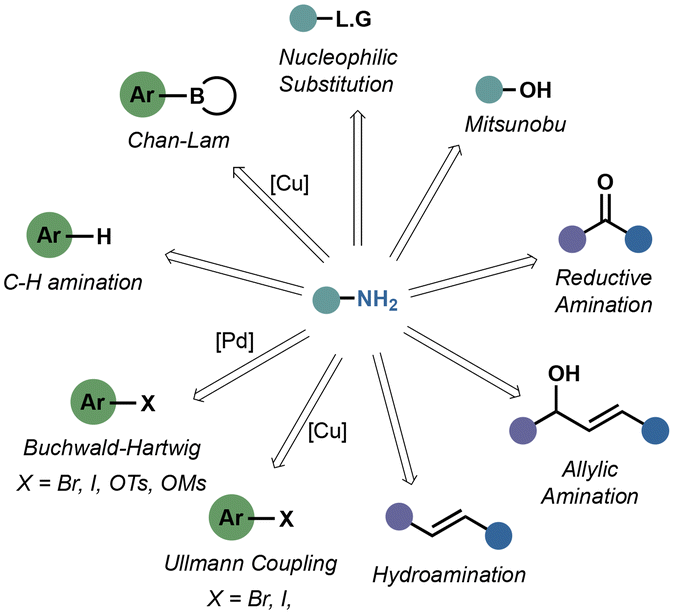
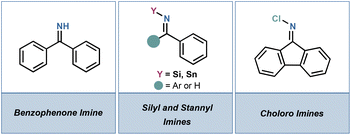
Ammonia surrogates in the synthesis of primary amines - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D3OB01202F

Tritylamine as an Ammonia Surrogate in the Ugi Reaction Provides Access to Unprecedented 5-Sulfamido Oxazoles Using Burgess-type Reagents
![PDF] Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction](https://d3i71xaburhd42.cloudfront.net/8c43d9c198206a16ca6c2f008f04220e840b4896/3-Table1-1.png)
PDF] Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction







