Strong and Weak Bases and Base Ionization Constant (Kb) Study Guide - Inspirit Learning Inc
4.5 (392) · $ 26.99 · In stock
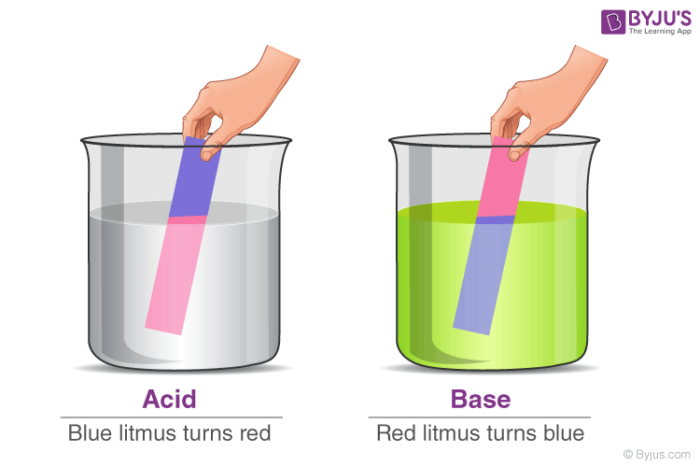
INTRODUCTION:You must have learned that when a litmus paper is placed in an acid or base, it changes color in your classes. This is also the indicating test to determine whether the given liquid is acid or base, or just water! Has it ever occurred to you what happens after identifying the given solution? Are

Strong and Weak Bases
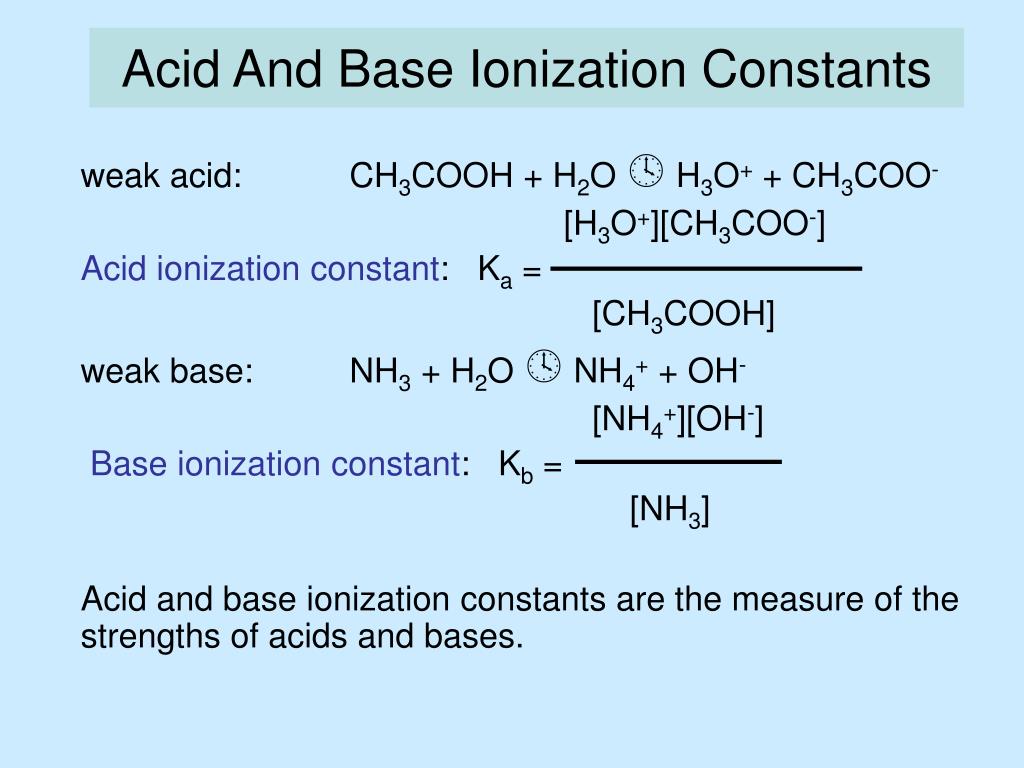
PPT - Acid-Base Strength: Ka, Kb, Kw PowerPoint Presentation, free download - ID:269976

Strength of Acids and Bases - Wize University Chemistry Textbook

Strong Base / Weak Acid Reactions - AP Chemistry Complete Course - Lesson 27.2
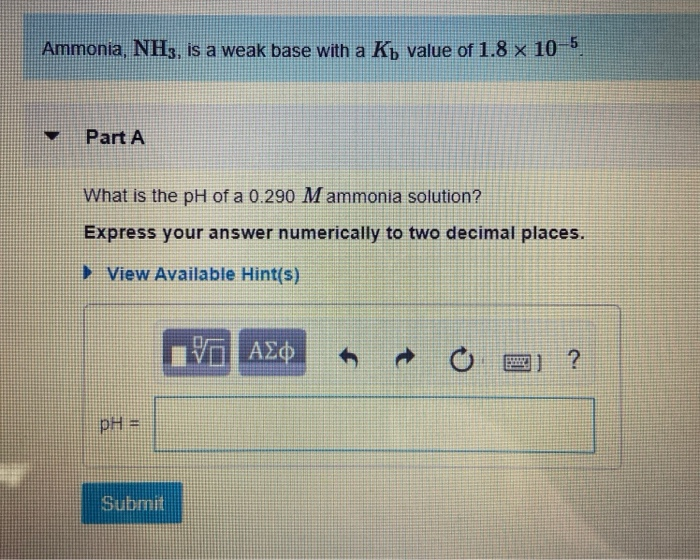
Solved The degree to which a weak base dissociates is given

PPT - Dissociation Constant or Ionization Constant for Weak Acids & Bases PowerPoint Presentation - ID:2437397

Equilibrium, Acids & Bases - ppt download

Chemistry - Acids & Bases (25 of 45) Ionization Constant for Weak Acids

Weak Acid Ionization Constants and the Determination of Weak Acid–Weak Base Reaction Equilibrium Constants in the General Chemistry Laboratory






![Base Set #3 [PNG, CSP, PSD]](https://www.chevistey.de/cdn/shop/products/image_6a88cda5-885a-4f56-9f79-024164243b0b_530x@2x.png?v=1659791298)
